Researchers at the Wyss Institute at Harvard University have found a way to trigger the self-assembly of tiny water-filled gel-like cubes into larger structures, a discovery that could lead to practical applications in tissue engineering.
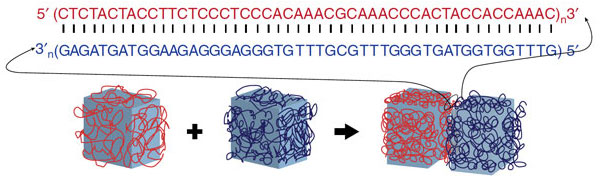
The scientists developed the self-assembling system by programming DNA to act as a glue that guides the hydrogels into the larger structures. Their results are published in the Sept. 9 issue of Nature Communications.
Researchers have attempted to program hydrogels in the past, but ran into trouble trying to bind them to other biological components, prompting the team at Wyss to devise a new strategy.
Using DNA as Glue
Enter DNA. It is made up of four bases — adenine, guanine, cytosine and thymine, or A, G, C and T. In order to form the coiled, double-helix structure of DNA, those bases have to be linked in a specific order: A with T and C with G. If one side of a strand of DNA should begin with AC, for example, then the corresponding rung would have to begin with TG.
Because snippets of DNA can be synthesized with any sequence of those letters, it is more programmable than other biomaterials, the Wyss researchers found. DNA can be, in effect, a glue. To test their theory, the researchers covered hydrogel cubes with a coat of a specific DNA base molecule.
When those small cubes were placed in a solution with larger cubes, the smaller ones attached only to cubes that were made up of their corresponding DNA base. Therefore, the scientists were able to program the hydrogels to mold into specific shapes, including a square and a T-shaped structure.
Eventually, the same method could potentially be used to create or repair more complex structures, including human tissue.
“This paper is a fundamental study of this capability, and it’s not quite ready for application yet, but we think this is a very promising direction for developing applications that could assemble these gel-like bricks into functional tissues,” Peng Yin, assistant professor of systems biology at Harvard Medical School and senior co-author of the study, told TechNewsWorld. “My colleagues and I hope to move forward together in this direction.”
Next Step
This research shows a potential solution for one of the main difficulties in tissue engineering — creating structures that go beyond two dimensions, said Robert Van Buskirk, professor in biological sciences at SUNY-Binghamton.
“This technology may prove to be critical for the next advance in tissue engineering,” he told TechNewsWorld.
The next steps in developing this research would be to conduct tests to determine how well the method could hold up in the sometimes unpredictable human body, said George Truskey, Ph.D., professor of biomedical engineering and senior associate dean for research at Duke University.
“The hydrogels will need to be populated with different cell types, and the investigators will need to show that self-assembly can induce different functions that might occur in a tissue,” he told TechNewsWorld. “The bigger challenge will be to establish that this approach will work in vivo, given the potential for enzymatic degradation of DNA and immunogenicity.”
It might be some time before researchers have answers about those questions, but the discovery is nonetheless a significant step forward in the field, Truskey noted. “This is an important achievement by a talented group that shows the approach is feasible.”